
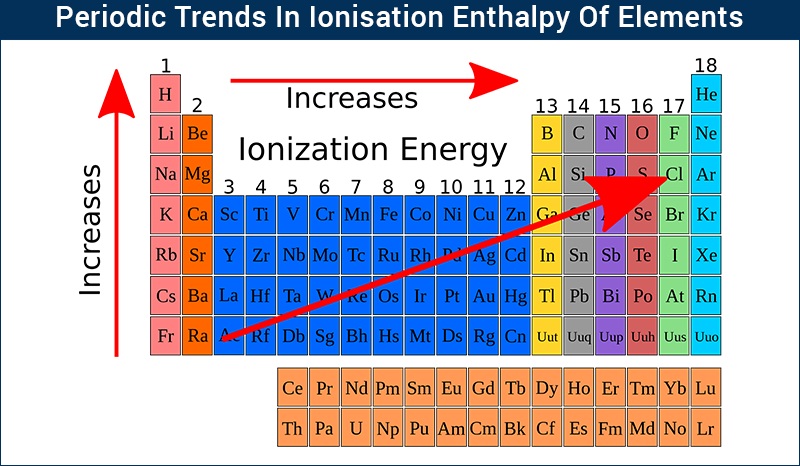

So far, you can rank the atomic radius of sulfur, chlorine, fluorine, and oxygen, in increasing order as: Sulfur and oxygen are in the same group (16), with sulfur directlly below oxygen, so sulfur the atomic radius of sulfur is larger than the atocmi radius of oxygen. For example, a sulfur atom (Ne3s23p4) has a covalent radius of 104 pm. That permits you to compare the size of the elements in a group:įluorine and chlorine are in the same group (17), with chlorine directly below fluorine, so the atomic radius of chlorine is larger than the atomic radius of fluorine. Cations with larger charges are smaller than cations with smaller charges (e.g. Atomic radius increases from top to bottom within a group due to electron shielding. Sulfur and chlorine are in the period 4, being sulfur to the left of chlorine, so sulfur is larger than chlorine. Among the elements of the main group, the first ionization energy increases. Which would least strongly attract electrons from other atoms in a compound elements of group 1. Oxygen and fluorine are in the period 3, being oxygen to the left of fluorine, so oxygen is larger than fluorine. The graph below shows the electronegativities of the elements in the periodic table. Hence, you can compare the elements that belong to a same period and predict that the atom with lower atomic number (number of protons) will haver larger atomic radius. Cadmium has larger atomic radius than sulfur.ĭown a period, atomic radii decrease from left to right due to the increase in the number of protons and electrons across a period: when a proton is added the pull of the electrons towards the nucleus is larger, so the size of the atom decreases.


 0 kommentar(er)
0 kommentar(er)
